New Publication: Fluorine-free dialkylphosphate-based ionic liquids as supercapacitor electrolytes
Energy Adv., 2025, Advance Article https://doi.org/10.1039/D5YA00217F
Title: Fluorine-free dialkylphosphate-based ionic liquids as supercapacitor electrolytes
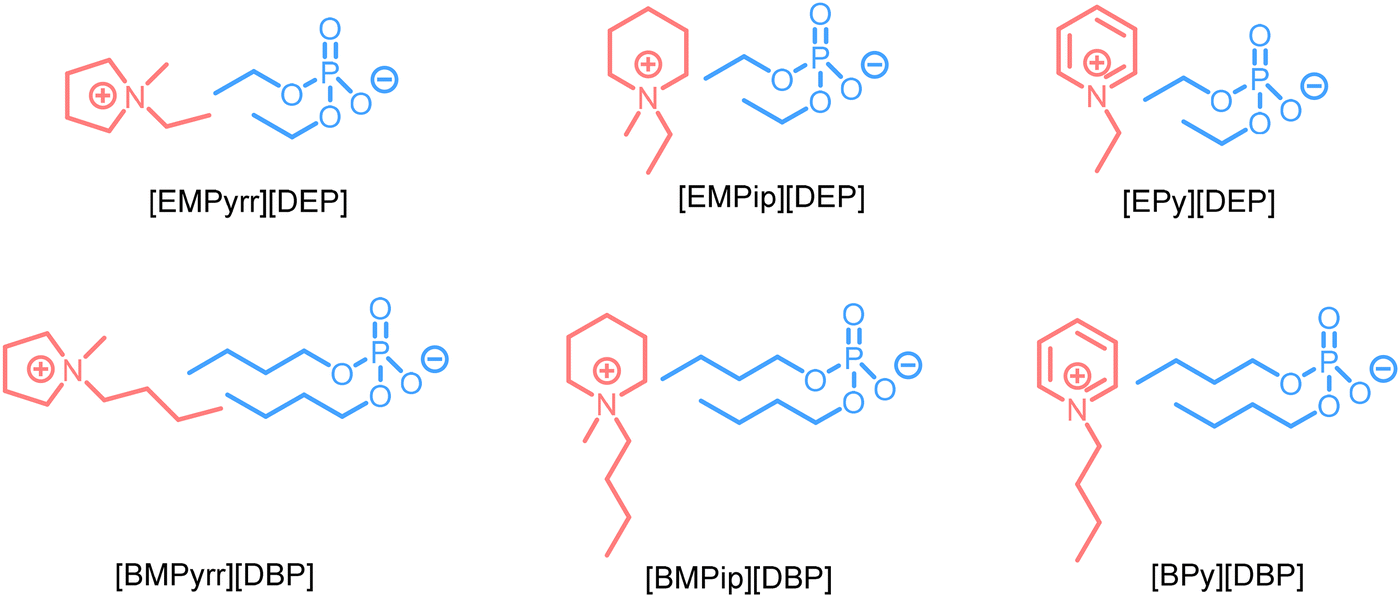
Abstract: The synthesis and physicochemical and electrochemical properties of several fluorine-free ionic liquids (ILs) comprising dialkylphosphate anions coupled to N-heterocyclic cations such as pyrrolidinium (Pyrr), piperidinium (Pip), and pyridinium (Py) are presented. All the ILs are synthesized in a single step by reacting trialkyl phosphates with pyrrolidine, piperidine, or pyridine. These ILs exhibit ionic conductivities in the range of 0.07 to 0.57 mS cm−1 at 20 °C, while increasing to 3.98 mS cm−1 at 60 °C, and an electrochemical stability window (ESW) up to 6.8 V on a glassy carbon (GC) electrode. Furthermore, a comparative performance of symmetric supercapacitors (SCs) made of multiwalled carbon nanotubes (MWCNTs) using [EMPyrr][DEP] and [BMPyrr][DBP] as electrolytes is presented. The SC based on [EMPyrr][DEP] reveals higher capacity retention, a power density of 1050 W kg−1, and an energy density of 68 Wh kg−1 using 0.5 A g−1 at 60 °C. This paves the way for developing fluorine-free and high-performant IL-based electrolytes for supercapacitors operating at elevated temperatures.